Abstract
Introduction:
Ponatinib is potently active against native and mutant BCR-ABL1, including T315I, and is approved for use in relapsed/intolerant patients with CML or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL), or those with the T315I mutation. The pivotal phase 2 PACE study (NCT01207440) assessed the safety and efficacy of ponatinib in patients with CML or Ph+ ALL resistant/intolerant to dasatinib or nilotinib, or with T315I.
Objective:
To evaluate whether patient characteristics and long-term outcomes with ponatinib differed by extent of pretreatment with other TKIs in CP-CML patients in PACE.
Methods:
Ponatinib was initiated at 45 mg once daily. Dose reductions were allowed for toxicity and mandated in Oct'13 due to observed arterial occlusive events (AOEs). In this post hoc analysis, baseline characteristics and long-term (~5 years median follow-up) efficacy and safety of ponatinib in CP-CML patients were evaluated according to previous treatment with 1, 2, 3, or 4 prior TKIs approved for use in CP-CML (ie, imatinib, dasatinib, nilotinib, and bosutinib). Final study data as of 6 Feb'17 are reported.
Results:
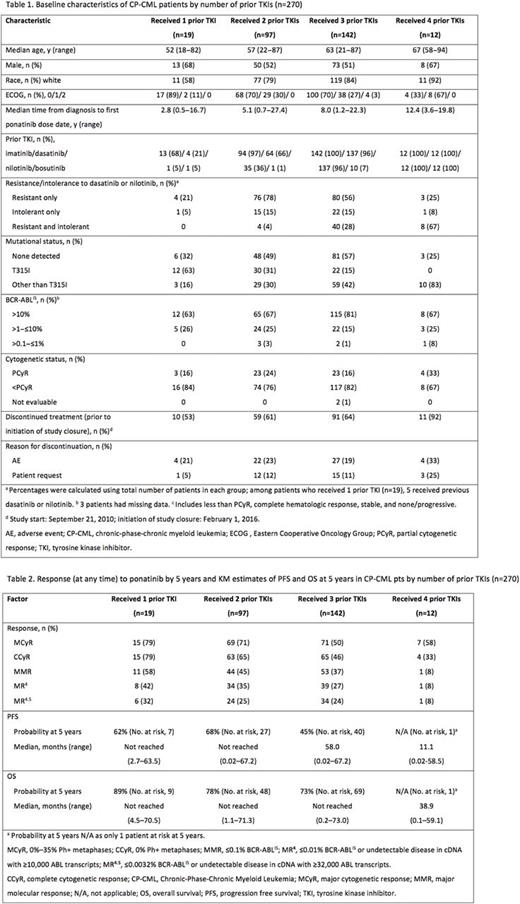
270 patients with CP-CML were treated and included in the current analysis (median follow-up: 56.8 months [range 0.1-73.1]). Overall, 60% received >3 prior TKIs. Patient characteristics and disposition varied by number of prior TKIs (Table 1). Median age at baseline and median time from diagnosis increased with increasing number of prior TKIs. Prior treatment resistance was more common than intolerance regardless of the number of prior TKIs. The proportion of patients who were both treatment resistant and intolerant to dasatinib or nilotinib increased with number of prior TKIs. Patients with the T315I mutation were more likely to have received only 1 or 2 prior TKIs vs 3 or 4; in contrast, patients with mutations other than T315I tended to have received more prior TKIs (Table 1). Median duration of ponatinib treatment decreased with number of prior TKIs, at 42.6, 38.4, 28.8, and 10.1 months with 1, 2, 3, or 4 prior TKIs, respectively; however, median dose intensity appeared higher in patients with 3 (28.7 mg/d) and 4 (31.0 mg/d) vs 1 (28.3 mg/d) and 2 (28.0 mg/d) prior TKIs. The most common reasons for discontinuation are shown in Table 1. Cumulative rates of cytogenetic and molecular response were higher with fewer prior TKIs (Table 2). Responses were durable with 1-3 prior TKIs (of those who achieved MCyR, Kaplan-Meier estimates for 5-year maintenance of response was 75%, 88%, and 84%, respectively), and less so with 4 prior TKIs (with 1 patient at risk at 5 years). Kaplan-Meier estimated PFS/OS rates at 5 years were high with 1-3 prior TKIs and lowest with 4 (Table 2). Overall, the most common any grade TEAEs were rash (47%), abdominal pain, thrombocytopenia (46% each), and headache (43%). When analyzed by prior TKIs received, the frequency of individual any grade TEAEs did not follow a consistent trend. However, the proportion of patients who reported at least 1 grade ≥3 TEAE increased with the number of prior TKIs (79% [15/19], 88% [85/97], 89% [127/142], and 100% [12/12], with 1, 2, 3, and 4 prior TKIs, respectively). Grade >3 AEs in >10% of CP-CML patients overall were thrombocytopenia (35%), neutropenia (17%), hypertension (14%), increased lipase (13%), abdominal pain (10%), and anemia (10%). The cumulative incidence rate of AOEs with 1, 2, 3, and 4 prior TKIs was 37%, 32%, 29%, and 42%, respectively, and the exposure-adjusted incidence rate of new treatment-emergent AOEs was 13.8, 13.1, 13.9, and 42.4 per 100 patient-years, respectively. As of the data cutoff, patients who discontinued treatment due to deaths (any cause) were 0% (0/19), 2.1% (2/97), 3.5% (5/142), and 16.7% (2/12) of patients who received 1, 2, 3, and 4 prior TKIs, respectively.
Conclusions:
With 5 years of follow-up of the PACE study, ponatinib continues to yield deep, durable, and clinically meaningful responses in CP-CML patients. This post hoc analysis by TKI treatment history indicates that CP-CML patients who had fewer prior TKIs appeared to exhibit better efficacy and safety profiles.
Hochhaus: Novartis: Research Funding; ARIAD: Research Funding; Pfizer: Research Funding; Incyte: Research Funding; BMS: Research Funding; MSD: Research Funding. Cortes: Pfizer: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; Sun Pharma: Research Funding; BMS: Consultancy, Research Funding; Teva: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding. Kim: BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Il-Yang: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Pinilla-Ibarz: BMS: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; ARIAD: Consultancy, Honoraria. le Coutre: BMS: Honoraria; Pfizer: Honoraria; Novartis: Honoraria, Research Funding; Incyte: Honoraria; ARIAD: Honoraria. Chuah: Chiltern: Honoraria; BMS: Honoraria, Other: Travel; Novartis: Honoraria; Avillion: Honoraria. Nicolini: Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Incyte Biosciences: Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; ARIAD: Honoraria, Speakers Bureau. Talpaz: Pfizer Inc: Consultancy, Other: Travel, Research Funding; ARIAD: Other: Travel, Research Funding. DeAngelo: Takeda Pharmaceuticals U.S.A., Inc.: Honoraria; Pfizer Inc.: Consultancy, Honoraria, Research Funding; BMS: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding; Glycomimetics: Research Funding; Celgene: Research Funding; Blueprint Medicines: Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Immunogen: Honoraria, Research Funding; Amgen: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; Shire: Honoraria. Abruzzese: Pfizer: Consultancy; Incyte: Consultancy; ARIAD: Consultancy; Novartis: Consultancy; Bristol Myers Squibb: Consultancy. Rea: Pfizer: Honoraria; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Incyte: Honoraria. Mueller: ARIAD: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; IHO GmbH: Equity Ownership. Gambacorti-Passerini: Pfizer: Consultancy, Honoraria, Research Funding; BMS: Consultancy. Castagnetti: Novartis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria. Milojkovic: Pfizer: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Incyte: Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; ARIAD: Consultancy, Honoraria. Lustgarten: ARIAD: Employment, Equity Ownership. Rivera: ARIAD: Employment, Equity Ownership. Neumann: Takeda Pharmaceuticals Inc: Employment, Equity Ownership. Guilhot: ARIAD: Honoraria. Deininger: Pfizer: Consultancy; Gilead: Research Funding; ARIAD: Consultancy; Ariad Pharmaceuticals, Bristol Myers Squibb, CTI BioPharma Corp, Gilead, Incyte, Novartis, Pfizer, Celgene, Blue Print, Galena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; Celgene: Research Funding; Incyte: Consultancy; BMS: Consultancy, Research Funding. Hughes: BMS: Consultancy, Honoraria, Research Funding; Ariad: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Shah: ARIAD: Research Funding; Daiichi-Sankyo: Research Funding; Pfizer: Research Funding; Bristol-Myers Squibb: Research Funding. Kantarjian: Amgen: Research Funding; Bristol-Meyers Squibb: Research Funding; Delta-Fly Pharma: Research Funding; ARIAD: Research Funding; Pfizer: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal